Feed Rate of Soda Ash in Wastewater
Alkalinity Adjustment
The use of the following chemicals will address Alkalinity adjustment issues in your plants.
- Magnesium Hydroxide,
- Lime Slurry
- Soda Ash
- Caustic Soda
With so many options for alkalinity adjustment, there are three main options that are typically represented in the marketplace for Wastewater Treatment Plants (WWTP) when faced with this question: magnesium hydroxide, lime slurry, and caustic soda. The following outlines the good and the bad of each, hopefully providing needed guidance for decision-making at WWTPs. Ultimately, the choice lies within the final requirements of the plant and driven by the discharge permit at the facility.
Most wastewater treatment plant operators understand that their wastewater treatment plants function best at some ideal pH and that a minimum amount of alkalinity is required to keep microorganisms happy. But too often, the values of pH and alkalinity are incorrectly used interchangeably, and a thorough understanding of each parameter's true relationship to biological stability and optimal performance – gets lost in the translation.
View Our pH & Alkalinity Adjuster Chemicals
Why is Alkalinity Important in Water Treatment?
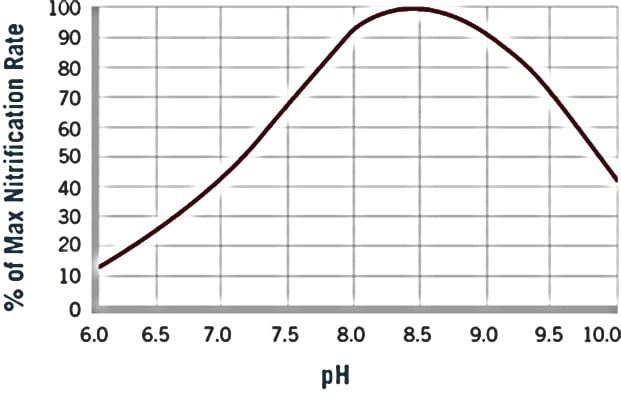 Most often, this error in terminology stems from the use of the most common alkaline pH modifiers and alkalinity supplements, caustic soda and lime. Where their use may successfully meet pH demands, they will likely fall short in supplying adequate alkalinity requirements without adversely elevating pH beyond biologically healthy limits. Furthermore, maintaining pH stability and uniformity across entire treatment basins remains a virtual impossibility. Therefore, it's important to look at each option for pH & alkalinity adjustments and ensure a good understanding of the effects of each on the system For example, when looking at nitrification process alone, it has been reported that having a stable, slightly elevated pH and enough alkalinity can drastically improve the denitrification of wastewater (see figure).
Most often, this error in terminology stems from the use of the most common alkaline pH modifiers and alkalinity supplements, caustic soda and lime. Where their use may successfully meet pH demands, they will likely fall short in supplying adequate alkalinity requirements without adversely elevating pH beyond biologically healthy limits. Furthermore, maintaining pH stability and uniformity across entire treatment basins remains a virtual impossibility. Therefore, it's important to look at each option for pH & alkalinity adjustments and ensure a good understanding of the effects of each on the system For example, when looking at nitrification process alone, it has been reported that having a stable, slightly elevated pH and enough alkalinity can drastically improve the denitrification of wastewater (see figure).

How do you treat alkalinity in water?
What chemical is used to increase alkalinity?
Common chemicals used to increase alkalinity and pH include:
- Calcium oxide or calcium hydroxide (as lime slurry)
- Sodium hydroxide (caustic soda)
- Sodium carbonate (soda ash) or sodium bicarbonate.
- Magnesium hydroxide or magnesium bicarbonate.
Hydrated Lime Slurry:
A very common product, also known as hydrated lime or CaO quicklime, lime can be 'slurry-ized' through the addition of water to dry lime powder. This slurry can be used to treat wastewater to increase pH and alkalinity and is commonly used in potable water to "soften," or remove hardness minerals, such as calcium and magnesium from drinking water.
Lime slurries minimize the effects of potential scaling in the water distribution system. It is also the most commonly used product to maintain alkalinity levels because of its low cost and multiple treatment benefits, such as assisting in the removal of manganese and iron from water.
The downside of lime slurry originates from its high solubility. Maintaining proper pH levels often becomes biologically prohibitive before ideal alkalinity levels and process stability can be reached. Another major concern is lime slurry will cause an increase in waste sludge, sometimes as much as 50% – adding more disposal costs to the operation. If that wasn't already enough, hydrated lime slurry added in collection systems increases the operations and maintenance costs related to formation of scale and accumulated solids/sludge. In severe cases this can lead to line blockages.
Caustic Soda:
Caustic Soda is commonly referred to Sodium Hydroxide or NaOH. Surprisingly, it can be commonly found in the home but in the industrial sense, it is mainly used for alkaline neutralization. Caustic Soda is found in all kinds of concentrations and is a common, popular way to neutralize and tame all kinds of acids. It is also considered easy to introduce to the system due to its solubility. However, at high concentrations, it is extremely hazardous to handle and several precautious must be in place to safely use in the treatment process. These would include enhanced PPE (personal protective equipment) and immediately accessible wash stations at a minimum.
Similar to Lime Slurry, because of the high solubility of Caustic Soda, pH often becomes biologically prohibitive very quickly. This often results in an increased risk of burning (elevated pH) out the microbiology before you can make adjustments. Additionally, the relationship between temperature and percentage of caustic in the solution is also important to pay attention to – above 50% concentration, the freeze point is 60°F restricting its use to warm climates or requiring heating tanks. Using lower percentage Caustic soda reduces the freezing point and diminishes some of these concerns, but you will need to pay more in freight to ship 'the water' content of the blend – ultimately a higher cost. Lastly, it is generally the most expensive of the three options discussed depending on current market value and delivery locations, especially when you take into account the relative dosage and alkalinity delivered per pound of product as discussed later.
Soda Ash:
Alkalinity and pH can be dramatically raised and controlled through the use of soda ash (sodium carbonate) where baking soda barely effects the overall pH. If you're trying to raise pH, Soda Ash is a the choice to use, under the guidance of the water treatment process.
Magnesium Hydroxide:
Magnesium Hydroxide is also referred to as milk of magnesia and the main function of the product is to neutralize the acids and stabilize alkalinity. Magnesium Hydroxide is generally worry free for alkalinity adjustments due to its self-buffering nature. Magnesium Hydroxide will only solubilize and generate a pH up to around 8.5, rendering this chemical safe to use because the likelihood of burning out (pH above 9) the biological activity is nearly impossible.
Magnesium Hydroxide does have some drawbacks. The slurry (typically 60-66%) separates and therefore must constantly be mixed or circulated. This issue can be readily solved and worry free through proper equipment selection to prevent this from happening. While Magnesium Hydroxide is the most difficult to store out of the three options presented, the benefits of alkalinity and pH stability will result in more consistent reaction times and with its self-buffering properties, will maintain more stable environments for wastewater treatment biological organisms to work as efficiently as possible – without killing them.

How Much Do You Need to Use?
Caustic Soda and Lime Slurry are definitely go to chemistries for the WWTP industry and they do provide the needed pH adjustments required. But on a pound to pound basis there is a fundamental difference between those two and magnesium hydroxide to also consider that does not have much to do with pH or alkalinity, but rather the true amount required to treat. For example, a given water sample may require 100 mg/L of magnesium hydroxide to raise the pH to 8.8. For the same sample, 138 mg/L of caustic soda and 135 mg/L of lime would be required.
To help control pH, we offer pHREADY, an effective alkalinity booster designed specifically to work in conjunction with our products to treat odor at the source! ChemREADY's Bio-Active manufacturing processes produce a superior, biologically receptive, nutrient and alkalinity source that makes it the product of choice for a diverse array of applications.
The Bottom Line Example
So, comparing magnesium hydroxide, caustic soda, and lime slurry, while all can supply the required benefits, the whole treatment process should be reviewed and determine the best overall solution based on some of the side effects for each. Magnesium hydroxide can be difficult to store when not done properly but can supply significantly more alkalinity in a bio-available form to a microbial wastewater system without adversely affecting pH. This creates a more suitable environment for bioremediation of BOD and nutrients like nitrogen and phosphorus. Moreover, because magnesium hydroxide supplies a lightweight, divalent cation, unlike the monovalent sodium in caustic and heavier calcium in lime, magnesium hydroxide helps to generate a denser, more easily dewatered sludge, with a higher percentage of cake solids – reducing waste disposal costs.
Source: https://www.getchemready.com/water-facts/what-should-i-use-for-alkalinity-adjustments-in-my-wastewater-treatment-plant/
0 Response to "Feed Rate of Soda Ash in Wastewater"
ارسال یک نظر